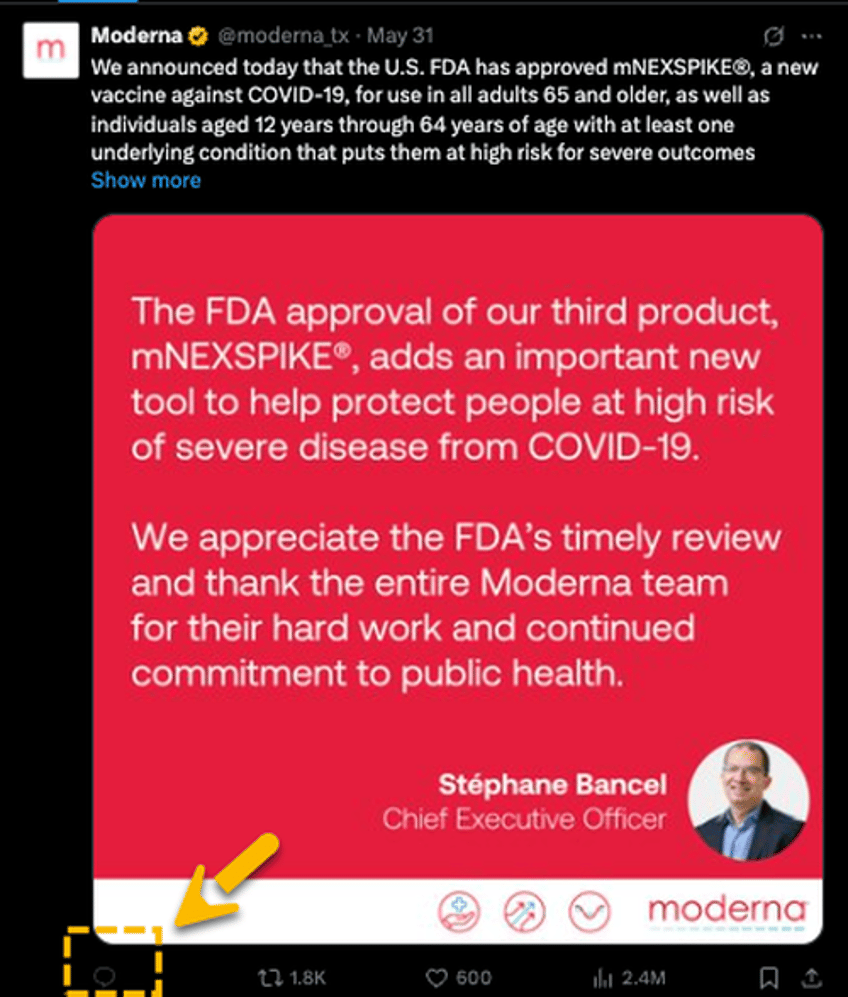
Moderna shares rose in premarket trading after the company announced FDA approval of its new lower-dose Covid-19 vaccine, mNEXSPIKE, for adults aged 65 and older, as well as individuals 12–64 with underlying health conditions. However, the approval sparked backlash on X, with many questioning the Trump administration and Health Secretary Robert F. Kennedy Jr.'s "MAHA" (Make America Healthy Again) agenda.
Moderna said in a Saturday morning X post that the mNEXSPIKE (mRNA-1283) vaccine should be available in the U.S. in time for the 2025-26 respiratory virus season, which starts later this year.
"The FDA approval of our third product, mNEXSPIKE, adds an important new tool to help protect people at high risk of severe disease from COVID-19," CEO Stéphane Bancel stated in a press release.
Bancel noted, "COVID-19 remains a serious public health threat, with more than 47,000 Americans dying from the virus last year alone. We appreciate the FDA's timely review and thank the entire Moderna team for their hard work and continued commitment to public health."
Last month, the FDA announced that Covid vaccines would not be offered this fall to healthy children and adults. At the same time, the Trump administration stated that any new Covid vaccines must undergo clinical trials using an inert placebo—such as saline—rather than being tested against existing approved vaccines.
Moderna's X post was met with heavy backlash, as many users voiced outrage over the FDA's approval, under Health Secretary RFK Jr., of yet another round of Covid vaccines.
Moderna's social media team disabled comments on the post. Bottom line: people are furious:
X user KAS14599753: "When your products are so safe and effective, you turn off the comments."
X user Dr Aseem Malhotra: "Complete and total madness. This company ( like Pfizer ) should be under investigation."
X user Gadsden2020: "Gee, I wonder why they don't let everyone comment on this? Could it be that they know people aren't buying their bullshit anymore? We know it's only about the $$$."
X user DocAhmadMalik: "Thank you, Donald Trump, Bobby, Jay, and Aseem, for proving that you are in on the game or totally hopeless."
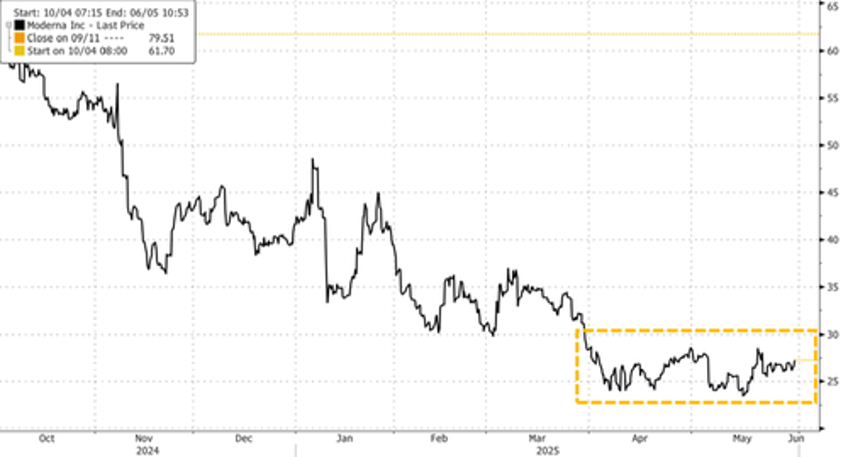
Despite the backlash on X, Wall Street analysts welcomed Moderna's new Covid vaccine, viewing it as a potential catalyst to lift the battered stock off its 52-week lows (list courtsey of Bloomberg):
Barclays, Gena Wang (equal weight)
Says the one-time approval removes a major overhang to the stock
"Label has no major surprise with patient population largely consistent with recent Covid framework"
"We would look for Phase 3 Flu data in summer to support Covid-Flu combo BLA re-submission and launch in 2026"
Leerink Partners, Mani Foroohar (underperform)
"With approval landing squarely in the bounds of the base case, we expect a modest relief rally on worst-case scenarios coming off the table"
Says attention on the vaccine pipeline now turns to Phase 3 flu vaccine efficacy interim data this summer, "which remains the key near-term catalyst for the stock"
William Blair, Myles R. Minter (market perform)
Says approval is an incremental win for Moderna "especially considering HHS Secretary Robert F. Kennedy Jr.'s negative public opinion on mRNA Covid-19 vaccines"
Does not see approval of the new vaccine as a massive boost to Moderna's Covid-19 vaccine sales, "which we believe are primarily driven by sentiment surrounding the vaccination market in general, but this is a critical step in the regulatory path for the combo flu/Covid vaccine product"
Shares were up more than 2% in premarket trading.
All you need to know: Moderna disabled comments on its X post—clearly unable to handle the wave of criticism over its new product.
As for Trump's MAHA agenda, X sentiment isn't thrilled—many are voicing frustration with RFK Jr.'s FDA and its latest vaccine approval. Why not approve alternative treatments?